Table of Contents
Triple-Negative Breast Cancer (TNBC) is one of the most aggressive types of breast cancer and remains a significant challenge in both diagnosis and treatment. This article provides an in-depth review of TNBC, examining its genetic and molecular characteristics, treatment options, the challenges faced in treating this cancer, and the latest advancements in therapeutic strategies. We will also explore ongoing clinical trials and provide insights into future treatment directions od TNBC.
Understanding Triple-Negative Breast Cancer (TNBC)
Triple-Negative Breast Cancer (TNBC) is a subtype of breast cancer defined by the absence of three critical biomarkers: estrogen receptor (ER), progesterone receptor (PR), and HER2. Because of this, TNBC is unresponsive to hormonal therapies that target the estrogen and progesterone receptors, as well as HER2-targeted treatments like trastuzumab (Herceptin). This unique feature of TNBC makes it particularly difficult to treat, requiring different therapeutic approaches compared to other types of breast cancer.
Genetic and Molecular Features of TNBC
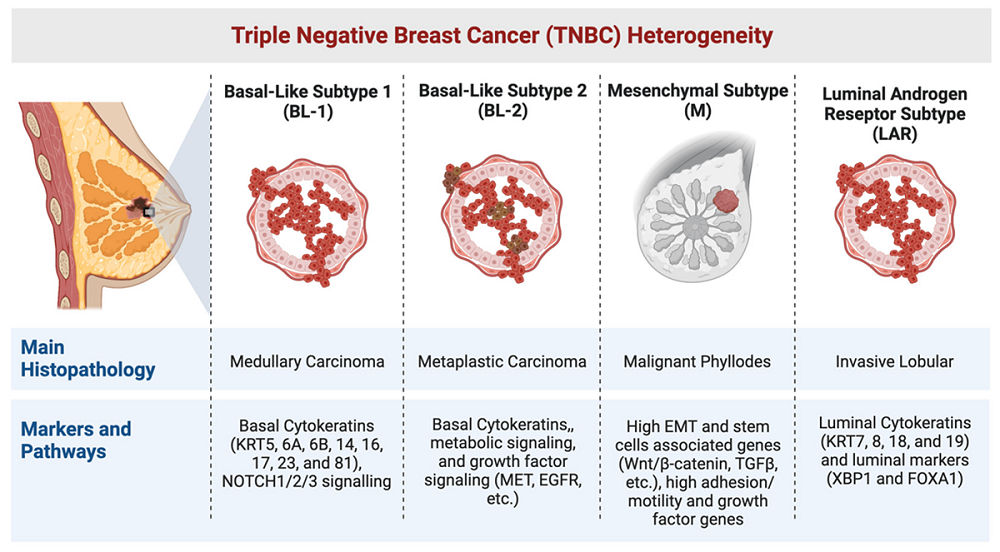
TNBC is genetically heterogeneous, which means it consists of several subtypes, each with distinct molecular features. Research has identified multiple genetic and molecular alterations associated with TNBC, including the basal-like subtype, which is the most common form of TNBC, and the mesenchymal-like subtype, which is more aggressive and resistant to conventional therapies. Understanding the molecular heterogeneity of TNBC is essential for developing personalized treatment strategies (Feng et al., 2018).
BRCA1 and BRCA2 gene mutations are strongly associated with TNBC. These mutations impair the DNA repair mechanisms in cells, leading to genomic instability, which contributes to cancer development. BRCA1 mutations are particularly linked with an earlier onset of TNBC and a higher risk of metastasis. In addition to these genetic mutations, TNBC cells often exhibit a high rate of chromosomal instability and a greater propensity for epithelial-to-mesenchymal transition (EMT), which facilitates metastasis (Li et al., 2021).
Risk Factors for TNBC
In addition to genetic mutations, several lifestyle and environmental factors increase the risk of developing TNBC. For instance, obesity, smoking, high alcohol consumption, and lack of physical activity have been shown to contribute to the risk of breast cancer, including TNBC. Ethnicity also plays a significant role, with TNBC being more prevalent among African American women and those of Ashkenazi Jewish descent due to higher rates of BRCA1/2 mutations.
TNBC Subtypes and Their Clinical Implications
The heterogeneity of TNBC is not only genetic but also functional. This means that TNBC tumors can behave very differently depending on their subtype. For example, basal-like TNBC is often more aggressive and has a higher likelihood of recurrence compared to other subtypes. On the other hand, luminal androgen receptor (LAR) positive TNBC, which expresses androgen receptors, may respond to hormonal therapies targeting the androgen receptor. Understanding these subtypes is critical for tailoring treatment strategies (Ortega et al., 2020).
Read: Food Poisoning vs Stomach Bug: Understanding the Differences
Challenges in Treating Triple-Negative Breast Cancer
The primary challenge in treating TNBC lies in its molecular characteristics, particularly the absence of ER, PR, and HER2 receptors. Without these receptors, traditional treatments like hormone therapy or HER2-targeted therapies, which have been effective in other breast cancer subtypes, cannot be used in TNBC treatment. This has made TNBC one of the most difficult breast cancer subtypes to treat effectively.
Chemotherapy as the Standard Treatment
Since TNBC lacks specific receptor targets, chemotherapy remains the cornerstone of treatment. Chemotherapy works by killing rapidly dividing cells, which is a hallmark of cancer. However, chemotherapy can also damage healthy cells, leading to significant side effects, including hair loss, nausea, and fatigue. In addition, as with all cancers, chemotherapy resistance can develop, rendering the treatment less effective over time.
Chemotherapy Drugs and Their Mechanism of Action
Common chemotherapy drugs used to treat TNBC include doxorubicin, cyclophosphamide, and paclitaxel. These drugs function by interfering with DNA replication and cell division, effectively slowing tumor growth. However, TNBC tumors are known to evolve quickly and may develop resistance to these drugs, particularly when the treatment is used in the long term (Fasching et al., 2016).
| Treatment Option | Mechanism of Action | Side Effects | Effectiveness |
|---|---|---|---|
| Chemotherapy | Inhibits cancer cell growth and division. | Hair loss, nausea, fatigue, infections | Effective initially, but resistance may develop. |
The Limitations of Chemotherapy
Despite being the standard treatment, chemotherapy has its limitations. It is not a specific treatment and can damage both cancerous and healthy cells, leading to a range of side effects. Additionally, as TNBC tumors can evolve rapidly, resistance to chemotherapy can develop, diminishing its effectiveness over time. This highlights the need for alternative and more targeted treatments for TNBC (Feng et al., 2018).
Emerging Therapeutic Approaches for TNBC
Immunotherapy: Revolutionizing Cancer Treatment
Immunotherapy has emerged as a promising treatment for TNBC in recent years. It works by harnessing the body’s immune system to recognize and attack cancer cells. Immune checkpoint inhibitors, such as pembrolizumab (Keytruda) and atezolizumab (Tecentriq), have been shown to improve survival outcomes in some TNBC patients when combined with chemotherapy (Medina et al., 2020).
Immune Checkpoint Inhibitors in TNBC
PD-1/PD-L1 inhibitors have been particularly effective in treating TNBC. Tumors with high PD-L1 expression tend to be more responsive to immunotherapy. This has led to the use of pembrolizumab and atezolizumab in combination with chemotherapy, which has significantly improved progression-free survival and overall survival in patients with advanced or metastatic TNBC (Won & Spruck, 2020).
| Treatment Option | Mechanism of Action | Side Effects | Effectiveness |
|---|---|---|---|
| Immunotherapy | Activates the immune system to target cancer cells. | Autoimmune diseases, skin rashes | More effective in PD-L1 positive patients. |
Challenges of Immunotherapy
While immunotherapy has shown significant promise, it is not universally effective. Only patients with PD-L1 positive tumors tend to benefit from immunotherapy, making it important to identify suitable candidates through genetic testing. Moreover, immune-related adverse effects, such as colitis and dermatitis, can occur, requiring careful monitoring during treatment (Li et al., 2021).
PARP Inhibitors: Targeting DNA Repair Mechanisms
PARP inhibitors, such as Olaparib (Lynparza), are particularly effective in patients with BRCA1 or BRCA2 mutations. These drugs block the enzyme PARP, which is responsible for repairing DNA damage. In patients with BRCA mutations, cancer cells are already deficient in DNA repair, making them particularly vulnerable to PARP inhibition.
How PARP Inhibitors Work
PARP inhibitors work by preventing the repair of DNA damage in cancer cells, leading to cell death. This approach is particularly effective in BRCA1 and BRCA2 mutation carriers, as their cells rely heavily on PARP for DNA repair. Clinical trials have shown that PARP inhibitors can improve progression-free survival in patients with metastatic TNBC (Li et al., 2021).
Read: Sustentaculum Tali 101: A Detailed Review
The Role of Personalized Medicine in TNBC Treatment
As our understanding of TNBC improves, the importance of personalized medicine in treatment becomes more apparent. Personalized medicine tailors treatment to the specific genetic profile of the patient’s tumor, allowing for more effective and targeted therapies.
Genetic Profiling and Biomarkers
Advances in genomic profiling have allowed researchers to identify several biomarkers that play a critical role in TNBC treatment. PD-L1 expression, VEGF (vascular endothelial growth factor), and PI3K/Akt/mTOR signaling pathways are important biomarkers used to guide treatment decisions. For example, patients with PD-L1 positive tumors may benefit more from immunotherapy than those with PD-L1 negative tumors (Ortega et al., 2020).
Future Directions in Personalized Treatment
The future of TNBC treatment will likely revolve around a more personalized approach, driven by advancements in genetic profiling and biomarker research. Understanding the molecular landscape of each patient’s tumor will enable clinicians to choose the most effective therapy for each individual, thus minimizing unnecessary side effects and improving therapeutic outcomes (Yadav et al., 2015). This could also lead to more targeted therapies and the development of drugs that specifically address the unique genetic mutations present in TNBC.
Key Clinical Trials and Research Studies
KEYNOTE-355 Study
The KEYNOTE-355 trial tested the combination of pembrolizumab (an immune checkpoint inhibitor) with chemotherapy. The results demonstrated improved survival rates in PD-L1 positive TNBC patients. This combination therapy has shown potential as a frontline treatment for advanced TNBC, especially in patients who do not respond well to traditional chemotherapy (Won & Spruck, 2020).
OlympiAD Trial
The OlympiAD trial focused on the use of Olaparib, a PARP inhibitor, in patients with BRCA1/2 mutations. The study demonstrated that Olaparib significantly improved progression-free survival in patients with BRCA1/2 mutations, offering a new, effective treatment option for genetically predisposed individuals with metastatic TNBC. This breakthrough highlights the importance of genetic testing in tailoring treatment plans for TNBC patients (Feng et al., 2018).
IMpassion130 and Atezolizumab
The IMpassion130 trial evaluated atezolizumab, an immune checkpoint inhibitor, in combination with chemotherapy in metastatic TNBC patients. The study found that the combination of atezolizumab and chemotherapy significantly improved overall survival, particularly in PD-L1 positive patients (Medina et al., 2020). This further emphasizes the growing role of immunotherapy in the treatment of TNBC, especially for patients with specific tumor markers.
The Role of Tumor Microenvironment in TNBC
Another critical factor in understanding TNBC and developing effective therapies is the tumor microenvironment. The tumor microenvironment consists of various cells, signaling molecules, and extracellular matrix components that interact with cancer cells. Research has shown that the tumor microenvironment plays a significant role in TNBC’s ability to metastasize and evade the immune system.
Immune Evasion and the Tumor Microenvironment
TNBC tumors often exhibit immune evasion mechanisms, which make it more difficult for the body’s immune system to target and destroy the cancer cells. One key player in this immune evasion is the PD-1/PD-L1 pathway, which helps the tumor cells escape immune surveillance by inhibiting the immune response. By targeting this pathway with immune checkpoint inhibitors like pembrolizumab and atezolizumab, researchers have begun to enhance the immune system’s ability to recognize and attack TNBC cells (Medina et al., 2020).
Therapeutic Targeting of the Tumor Microenvironment
Researchers are also exploring strategies to modify the tumor microenvironment to make it more conducive to immune cell activity. This could involve using angiogenesis inhibitors like bevacizumab to reduce the tumor’s blood supply, or targeting the tumor-associated macrophages (TAMs) that often support tumor growth and immune evasion. By disrupting these components of the microenvironment, therapies may enhance the effectiveness of traditional treatments like chemotherapy and immunotherapy.
Advances in CAR-T Cell Therapy for TNBC
Chimeric Antigen Receptor T-Cell (CAR-T) therapy is a revolutionary treatment that involves genetically modifying a patient’s T-cells to better recognize and attack cancer cells. Although CAR-T therapy has been highly successful in treating hematologic malignancies such as leukemia and lymphoma, its application to solid tumors like TNBC has been more challenging.
CAR-T Cell Therapy: Potential for TNBC
CAR-T therapy for TNBC is still in the early stages of development, but initial preclinical studies have shown promising results. In these studies, CAR-T cells have been engineered to target specific tumor antigens present in TNBC, such as HER2, which is overexpressed in a subset of TNBC tumors. Clinical trials are ongoing to evaluate the safety and efficacy of CAR-T cell therapy for TNBC, with early results showing that it may be a viable treatment for patients with HER2 positive TNBC or other antigenic targets (Copeland & Kanaan, 2021).
Looking Ahead: The Future of TNBC Treatment
The future of TNBC treatment is evolving, with many exciting prospects on the horizon. The integration of immunotherapy, targeted therapies, and personalized medicine will continue to improve patient outcomes, making treatment more effective and tailored to each patient’s unique tumor profile.
Genetic Testing and Personalized Treatment
Genetic testing is becoming an increasingly important tool in the management of TNBC. By identifying BRCA mutations, PD-L1 expression, and other biomarkers, doctors can better predict which treatments will be most effective for individual patients. This allows for more targeted therapies that minimize side effects and improve the overall response to treatment (Yadav et al., 2015).
Combination Therapies for Enhanced Efficacy
Combining therapies like chemotherapy, immunotherapy, and PARP inhibitors may lead to more successful outcomes for TNBC patients. Research is ongoing to identify the best combinations of these treatments, particularly in patients who have failed standard chemotherapy (Feng et al., 2018). Such approaches are expected to increase progression-free survival and reduce recurrence rates, providing better long-term outcomes for TNBC patients.
The Role of Clinical Trials in Shaping the Future
Clinical trials will remain essential in shaping the future of TNBC treatment. Ongoing studies evaluating new drug combinations, immune therapies, and cutting-edge technologies will likely provide new insights and pave the way for more effective treatment options. As these trials progress, we can expect to see better-targeted therapies with fewer side effects and improved survival rates for patients with TNBC.
Conclusion
Triple-Negative Breast Cancer (TNBC) continues to present significant challenges in terms of treatment due to the lack of targeted therapies. However, advances in immunotherapy, PARP inhibitors, and CAR-T cell therapy provide hope for improved outcomes in the future. Additionally, personalized medicine is transforming the treatment landscape, enabling doctors to tailor therapies to the genetic profiles of individual patients. As research continues to progress, the outlook for TNBC patients will continue to improve, with new therapies offering more targeted and effective treatment options.
References
- Feng, Y., Spezia, M., Huang, S., Yuan, C., Zeng, Z., Zhang, L., Ji, X., Liu, W., Huang, B., Luo, W., Liu, B., Lei, Y., Du, S., Vuppalapati, A., Luu, H. H., Haydon, R. C., He, T. C., & Ren, G. (2018). Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases, 5(2), 77–106. https://doi.org/10.1016/J.GENDIS.2018.05.001
- Fasching, P. A., Gaß, P., & Hein, A. (2016). Neoadjuvant Treatment of Breast Cancer-Advances and Limitations. Breast Care, 11, 313–314. https://doi.org/10.1159/000452463
- Li, Y., Zhan, Z., Yin, X., Fu, S., & Deng, X. (2021). Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Frontiers in Oncology. https://doi.org/10.3389/fonc.2021.731535
- Won, K. A., & Spruck, C. (2020). Triple-negative breast cancer therapy: Current and future perspectives. International Journal of Oncology, 57(6), 1245–1261. https://doi.org/10.3892/IJO.2020.5135/HTML
- Ortega, M. A., Fraile-Martínez, O., Asúnsolo, A., Buján, J., García-Honduvilla, N., Coca, S., & Grzybowska-Szatkowska, L. (2020). Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. BioMed Research International. https://doi.org/10.1155/2020/9258396
- Medina, M. A., Oza, G., Sharma, A., Arriaga, L. G., Manuel Hernández Hernández, J., Rotello, V. M., & Tapia Ramirez, J. (2020). Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. International Journal of Environmental Research and Public Health, 17(6), 2078. https://doi.org/10.3390/ijerph17062078
- Yadav, B. S., Chanana, P., & Jhamb, S. (2015). Biomarkers in triple negative breast cancer: A review. World Journal of Clinical Oncology, 6(6), 252–263. https://doi.org/10.5306/WJCO.V6.I6.252
- Copeland, R. L., & Kanaan, Y. (2021). New targets in triple-negative breast cancer. Nature Reviews Cancer, 21(12), 744–744. https://doi.org/10.1038/s41568-02100415-4
- Hwang, S. Y., Park, S., & Kwon, Y. (2019). Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacology and Therapeutics, 199, 30–57. https://doi.org/10.1016/J.PHARMTHERA.2019.02.006
- Li, Y., Zhan, Z., Yin, X., Fu, S., & Deng, X. (2021). Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Frontiers in Oncology. https://doi.org/10.3389/fonc.2021.731535
- Medina, M. A., Oza, G., Sharma, A., Arriaga, L. G., Manuel Hernández Hernández, J., Rotello, V. M., & Tapia Ramirez, J. (2020). Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. International Journal of Environmental Research and Public Health, 17(6), 2078. https://doi.org/10.3390/ijerph17062078
- Copeland, R. L., & Kanaan, Y. (2021). New targets in triple-negative breast cancer. Nature Reviews Cancer, 21(12), 744–744. https://doi.org/10.1038/s41568-02100415-4
- Li, Y., Zhan, Z., Yin, X., Fu, S., & Deng, X. (2021). Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Frontiers in Oncology. https://doi.org/10.3389/fonc.2021.731535

